Tidy Transcriptomics for Single-cell RNA Sequencing Analyses with Bioconductor
Maria Doyle, Peter MacCallum Cancer Centre1
Stefano Mangiola, Walter and Eliza Hall Institute2
Source:vignettes/single_cell_bioconductor_transcriptomics.Rmd
single_cell_bioconductor_transcriptomics.RmdInstructors
Dr. Stefano Mangiola is currently a Postdoctoral researcher in the laboratory of Prof. Tony Papenfuss at the Walter and Eliza Hall Institute in Melbourne, Australia. His background spans from biotechnology to bioinformatics and biostatistics. His research focuses on prostate and breast tumour microenvironment, the development of statistical models for the analysis of RNA sequencing data, and data analysis and visualisation interfaces.
Workshop goals and objectives
What you will learn
- Basic
tidyoperations possible withtidyseuratandtidySingleCellExperiment - The differences between
SeuratandSingleCellExperimentrepresentation, andtidyrepresentation - How to interface
SeuratandSingleCellExperimentwith tidy manipulation and visualisation - A real-world case study that will showcase the power of
tidysingle-cell methods compared with base/ad-hoc methods
Part 1 Introduction to tidySingleCellExperiment
# Load packages
library(SingleCellExperiment)
library(ggplot2)
library(plotly)
library(dplyr)
library(colorspace)
library(dittoSeq)SingleCellExperiment is a very popular analysis toolkit for single cell RNA sequencing data (Butler et al. 2018; Stuart et al. 2019).
Here we load single-cell data in SingleCellExperiment object format. This data is peripheral blood mononuclear cells (PBMCs) from metastatic breast cancer patients.
# load single cell RNA sequencing data
sce_obj <- tidyomicsWorkshop::sce_obj
# take a look
sce_obj## class: SingleCellExperiment
## dim: 482 3580
## metadata(0):
## assays(2): counts logcounts
## rownames(482): HBB HBA2 ... TRGC2 CD8A
## rowData names(0):
## colnames(3580): 1_AAATGGACAAGTTCGT-1 1_AACAAGAGTGTTGAGG-1 ...
## 10_TTTGTTGGTACACGTT-1 10_TTTGTTGGTGATAGAT-1
## colData names(15): Barcode batch ... treatment sample
## reducedDimNames(1): UMAP
## mainExpName: SCT
## altExpNames(1): RNAtidySingleCellExperiment provides a bridge between the SingleCellExperiment single-cell package and the tidyverse (Wickham et al. 2019). It creates an invisible layer that enables viewing the SingleCellExperiment object as a tidyverse tibble, and provides SingleCellExperiment-compatible dplyr, tidyr, ggplot and plotly functions.
If we load the tidySingleCellExperiment package and then view the single cell data, it now displays as a tibble.
library(tidySingleCellExperiment)
sce_obj## # A SingleCellExperiment-tibble abstraction: 3,580 × 18
## # Features=482 | Cells=3580 | Assays=counts, logcounts
## .cell Barcode batch BCB S.Score G2M.Score Phase cell_type nCount_RNA
## <chr> <fct> <fct> <fct> <dbl> <dbl> <fct> <fct> <int>
## 1 1_AAATGGAC… AAATGG… 1 BCB1… -2.07e-2 -0.0602 G1 TCR_V_De… 215
## 2 1_AACAAGAG… AACAAG… 1 BCB1… 2.09e-2 -0.0357 S CD8+_tra… 145
## 3 1_AACGTCAG… AACGTC… 1 BCB1… -2.54e-2 -0.133 G1 CD8+_hig… 356
## 4 1_AACTTCTC… AACTTC… 1 BCB1… 2.85e-2 -0.163 S CD8+_tra… 385
## 5 1_AAGTCGTG… AAGTCG… 1 BCB1… -2.30e-2 -0.0581 G1 MAIT 352
## 6 1_AATGAAGC… AATGAA… 1 BCB1… 1.09e-2 -0.0621 S CD4+_rib… 335
## 7 1_ACAAAGAG… ACAAAG… 1 BCB1… -2.06e-2 -0.0409 G1 CD4+_Tcm… 242
## 8 1_ACACGCGC… ACACGC… 1 BCB1… -3.95e-4 -0.176 G1 CD8+_hig… 438
## 9 1_ACATGCAT… ACATGC… 1 BCB1… -4.09e-2 -0.0829 G1 CD4+_rib… 180
## 10 1_ACGATCAT… ACGATC… 1 BCB1… 8.82e-2 -0.0397 S CD4+_rib… 82
## # ℹ 3,570 more rows
## # ℹ 9 more variables: nFeature_RNA <int>, nCount_SCT <int>, nFeature_SCT <int>,
## # ident <fct>, file <chr>, treatment <chr>, sample <glue>, UMAP_1 <dbl>,
## # UMAP_2 <dbl>If we want to revert to the standard SingleCellExperiment view we can do that.
options("restore_SingleCellExperiment_show" = TRUE)
sce_obj## class: SingleCellExperiment
## dim: 482 3580
## metadata(0):
## assays(2): counts logcounts
## rownames(482): HBB HBA2 ... TRGC2 CD8A
## rowData names(0):
## colnames(3580): 1_AAATGGACAAGTTCGT-1 1_AACAAGAGTGTTGAGG-1 ...
## 10_TTTGTTGGTACACGTT-1 10_TTTGTTGGTGATAGAT-1
## colData names(15): Barcode batch ... treatment sampleIf we want to revert back to tidy SingleCellExperiment view we can.
options("restore_SingleCellExperiment_show" = FALSE)
sce_obj## # A SingleCellExperiment-tibble abstraction: 3,580 × 18
## # Features=482 | Cells=3580 | Assays=counts, logcounts
## .cell Barcode batch BCB S.Score G2M.Score Phase cell_type nCount_RNA
## <chr> <fct> <fct> <fct> <dbl> <dbl> <fct> <fct> <int>
## 1 1_AAATGGAC… AAATGG… 1 BCB1… -2.07e-2 -0.0602 G1 TCR_V_De… 215
## 2 1_AACAAGAG… AACAAG… 1 BCB1… 2.09e-2 -0.0357 S CD8+_tra… 145
## 3 1_AACGTCAG… AACGTC… 1 BCB1… -2.54e-2 -0.133 G1 CD8+_hig… 356
## 4 1_AACTTCTC… AACTTC… 1 BCB1… 2.85e-2 -0.163 S CD8+_tra… 385
## 5 1_AAGTCGTG… AAGTCG… 1 BCB1… -2.30e-2 -0.0581 G1 MAIT 352
## 6 1_AATGAAGC… AATGAA… 1 BCB1… 1.09e-2 -0.0621 S CD4+_rib… 335
## 7 1_ACAAAGAG… ACAAAG… 1 BCB1… -2.06e-2 -0.0409 G1 CD4+_Tcm… 242
## 8 1_ACACGCGC… ACACGC… 1 BCB1… -3.95e-4 -0.176 G1 CD8+_hig… 438
## 9 1_ACATGCAT… ACATGC… 1 BCB1… -4.09e-2 -0.0829 G1 CD4+_rib… 180
## 10 1_ACGATCAT… ACGATC… 1 BCB1… 8.82e-2 -0.0397 S CD4+_rib… 82
## # ℹ 3,570 more rows
## # ℹ 9 more variables: nFeature_RNA <int>, nCount_SCT <int>, nFeature_SCT <int>,
## # ident <fct>, file <chr>, treatment <chr>, sample <glue>, UMAP_1 <dbl>,
## # UMAP_2 <dbl>It can be interacted with using SingleCellExperiment
commands such as assays.
assays(sce_obj)## List of length 2
## names(2): counts logcountsWe can also interact with our object as we do with any tidyverse tibble.
Tidyverse commands
We can use tidyverse commands, such as filter,
select and mutate to explore the
tidySingleCellExperiment object. Some examples are shown below and more
can be seen at the tidySingleCellExperiment website here.
We can use filter to choose rows, for example, to see
just the rows for the cells in G1 cell-cycle stage.
sce_obj |> filter(Phase == "G1")## # A SingleCellExperiment-tibble abstraction: 1,859 × 18
## # Features=482 | Cells=1859 | Assays=counts, logcounts
## .cell Barcode batch BCB S.Score G2M.Score Phase cell_type nCount_RNA
## <chr> <fct> <fct> <fct> <dbl> <dbl> <fct> <fct> <int>
## 1 1_AAATGGAC… AAATGG… 1 BCB1… -2.07e-2 -0.0602 G1 TCR_V_De… 215
## 2 1_AACGTCAG… AACGTC… 1 BCB1… -2.54e-2 -0.133 G1 CD8+_hig… 356
## 3 1_AAGTCGTG… AAGTCG… 1 BCB1… -2.30e-2 -0.0581 G1 MAIT 352
## 4 1_ACAAAGAG… ACAAAG… 1 BCB1… -2.06e-2 -0.0409 G1 CD4+_Tcm… 242
## 5 1_ACACGCGC… ACACGC… 1 BCB1… -3.95e-4 -0.176 G1 CD8+_hig… 438
## 6 1_ACATGCAT… ACATGC… 1 BCB1… -4.09e-2 -0.0829 G1 CD4+_rib… 180
## 7 1_ACGTAACG… ACGTAA… 1 BCB1… -8.69e-3 -0.140 G1 TCR_V_De… 187
## 8 1_AGAGAATT… AGAGAA… 1 BCB1… -5.14e-3 -0.102 G1 CD8+_tra… 155
## 9 1_AGATCCAT… AGATCC… 1 BCB1… -1.17e-2 -0.155 G1 CD8+_hig… 433
## 10 1_AGCCAATA… AGCCAA… 1 BCB1… -3.61e-2 -0.207 G1 CD4+_Tcm… 353
## # ℹ 1,849 more rows
## # ℹ 9 more variables: nFeature_RNA <int>, nCount_SCT <int>, nFeature_SCT <int>,
## # ident <fct>, file <chr>, treatment <chr>, sample <glue>, UMAP_1 <dbl>,
## # UMAP_2 <dbl>We can use select to view columns, for example, to see
the filename, total cellular RNA abundance and cell phase.
- If we use
selectwe will also get any view-only columns returned, such as the UMAP columns generated during the preprocessing.
sce_obj |> select(.cell, file, nCount_RNA, Phase)## # A SingleCellExperiment-tibble abstraction: 3,580 × 6
## # Features=482 | Cells=3580 | Assays=counts, logcounts
## .cell file nCount_RNA Phase UMAP_1 UMAP_2
## <chr> <chr> <int> <fct> <dbl> <dbl>
## 1 1_AAATGGACAAGTTCGT-1 ../data/single_cell/SI-G… 215 G1 -3.73 -1.59
## 2 1_AACAAGAGTGTTGAGG-1 ../data/single_cell/SI-G… 145 S 0.798 -0.151
## 3 1_AACGTCAGTCTATGAC-1 ../data/single_cell/SI-G… 356 G1 -0.292 0.515
## 4 1_AACTTCTCACGCTGAC-1 ../data/single_cell/SI-G… 385 S 0.372 2.34
## 5 1_AAGTCGTGTGTTGCCG-1 ../data/single_cell/SI-G… 352 G1 -1.63 -0.236
## 6 1_AATGAAGCATCCAACA-1 ../data/single_cell/SI-G… 335 S 0.822 2.90
## 7 1_ACAAAGAGTCGTACTA-1 ../data/single_cell/SI-G… 242 G1 3.28 3.97
## 8 1_ACACGCGCAGGTACGA-1 ../data/single_cell/SI-G… 438 G1 -3.65 -0.192
## 9 1_ACATGCATCACTTTGT-1 ../data/single_cell/SI-G… 180 G1 -0.273 4.09
## 10 1_ACGATCATCGACGAGA-1 ../data/single_cell/SI-G… 82 S -0.816 2.90
## # ℹ 3,570 more rowsWe can use mutate to create a column. For example, we
could create a new Phase_l column that contains a
lower-case version of Phase.
## # A SingleCellExperiment-tibble abstraction: 3,580 × 5
## # Features=482 | Cells=3580 | Assays=counts, logcounts
## .cell Phase Phase_l UMAP_1 UMAP_2
## <chr> <fct> <chr> <dbl> <dbl>
## 1 1_AAATGGACAAGTTCGT-1 G1 g1 -3.73 -1.59
## 2 1_AACAAGAGTGTTGAGG-1 S s 0.798 -0.151
## 3 1_AACGTCAGTCTATGAC-1 G1 g1 -0.292 0.515
## 4 1_AACTTCTCACGCTGAC-1 S s 0.372 2.34
## 5 1_AAGTCGTGTGTTGCCG-1 G1 g1 -1.63 -0.236
## 6 1_AATGAAGCATCCAACA-1 S s 0.822 2.90
## 7 1_ACAAAGAGTCGTACTA-1 G1 g1 3.28 3.97
## 8 1_ACACGCGCAGGTACGA-1 G1 g1 -3.65 -0.192
## 9 1_ACATGCATCACTTTGT-1 G1 g1 -0.273 4.09
## 10 1_ACGATCATCGACGAGA-1 S s -0.816 2.90
## # ℹ 3,570 more rowsWe can use tidyverse commands to polish an annotation column. We will extract the sample, and group information from the file name column into separate columns.
# First take a look at the file column
sce_obj |> select(.cell, file)## # A SingleCellExperiment-tibble abstraction: 3,580 × 4
## # Features=482 | Cells=3580 | Assays=counts, logcounts
## .cell file UMAP_1 UMAP_2
## <chr> <chr> <dbl> <dbl>
## 1 1_AAATGGACAAGTTCGT-1 ../data/single_cell/SI-GA-H1/outs/raw_fea… -3.73 -1.59
## 2 1_AACAAGAGTGTTGAGG-1 ../data/single_cell/SI-GA-H1/outs/raw_fea… 0.798 -0.151
## 3 1_AACGTCAGTCTATGAC-1 ../data/single_cell/SI-GA-H1/outs/raw_fea… -0.292 0.515
## 4 1_AACTTCTCACGCTGAC-1 ../data/single_cell/SI-GA-H1/outs/raw_fea… 0.372 2.34
## 5 1_AAGTCGTGTGTTGCCG-1 ../data/single_cell/SI-GA-H1/outs/raw_fea… -1.63 -0.236
## 6 1_AATGAAGCATCCAACA-1 ../data/single_cell/SI-GA-H1/outs/raw_fea… 0.822 2.90
## 7 1_ACAAAGAGTCGTACTA-1 ../data/single_cell/SI-GA-H1/outs/raw_fea… 3.28 3.97
## 8 1_ACACGCGCAGGTACGA-1 ../data/single_cell/SI-GA-H1/outs/raw_fea… -3.65 -0.192
## 9 1_ACATGCATCACTTTGT-1 ../data/single_cell/SI-GA-H1/outs/raw_fea… -0.273 4.09
## 10 1_ACGATCATCGACGAGA-1 ../data/single_cell/SI-GA-H1/outs/raw_fea… -0.816 2.90
## # ℹ 3,570 more rows
# Create column for sample
sce_obj <- sce_obj |>
# Extract sample
extract(file, "sample", "../data/.*/([a-zA-Z0-9_-]+)/outs.+", remove = FALSE)
# Take a look
sce_obj |> select(.cell, sample, everything())## # A SingleCellExperiment-tibble abstraction: 3,580 × 18
## # Features=482 | Cells=3580 | Assays=counts, logcounts
## .cell sample Barcode batch BCB S.Score G2M.Score Phase cell_type
## <chr> <chr> <fct> <fct> <fct> <dbl> <dbl> <fct> <fct>
## 1 1_AAATGGACAAGT… SI-GA… AAATGG… 1 BCB1… -2.07e-2 -0.0602 G1 TCR_V_De…
## 2 1_AACAAGAGTGTT… SI-GA… AACAAG… 1 BCB1… 2.09e-2 -0.0357 S CD8+_tra…
## 3 1_AACGTCAGTCTA… SI-GA… AACGTC… 1 BCB1… -2.54e-2 -0.133 G1 CD8+_hig…
## 4 1_AACTTCTCACGC… SI-GA… AACTTC… 1 BCB1… 2.85e-2 -0.163 S CD8+_tra…
## 5 1_AAGTCGTGTGTT… SI-GA… AAGTCG… 1 BCB1… -2.30e-2 -0.0581 G1 MAIT
## 6 1_AATGAAGCATCC… SI-GA… AATGAA… 1 BCB1… 1.09e-2 -0.0621 S CD4+_rib…
## 7 1_ACAAAGAGTCGT… SI-GA… ACAAAG… 1 BCB1… -2.06e-2 -0.0409 G1 CD4+_Tcm…
## 8 1_ACACGCGCAGGT… SI-GA… ACACGC… 1 BCB1… -3.95e-4 -0.176 G1 CD8+_hig…
## 9 1_ACATGCATCACT… SI-GA… ACATGC… 1 BCB1… -4.09e-2 -0.0829 G1 CD4+_rib…
## 10 1_ACGATCATCGAC… SI-GA… ACGATC… 1 BCB1… 8.82e-2 -0.0397 S CD4+_rib…
## # ℹ 3,570 more rows
## # ℹ 9 more variables: nCount_RNA <int>, nFeature_RNA <int>, nCount_SCT <int>,
## # nFeature_SCT <int>, ident <fct>, file <chr>, treatment <chr>, UMAP_1 <dbl>,
## # UMAP_2 <dbl>We could use tidyverse unite to combine columns, for
example to create a new column for sample id combining the sample and
patient id (BCB) columns.
sce_obj <- sce_obj |> unite("sample_id", sample, BCB, remove = FALSE)
# Take a look
sce_obj |> select(.cell, sample_id, sample, BCB)## # A SingleCellExperiment-tibble abstraction: 3,580 × 6
## # Features=482 | Cells=3580 | Assays=counts, logcounts
## .cell sample_id sample BCB UMAP_1 UMAP_2
## <chr> <chr> <chr> <fct> <dbl> <dbl>
## 1 1_AAATGGACAAGTTCGT-1 SI-GA-H1_BCB102 SI-GA-H1 BCB102 -3.73 -1.59
## 2 1_AACAAGAGTGTTGAGG-1 SI-GA-H1_BCB102 SI-GA-H1 BCB102 0.798 -0.151
## 3 1_AACGTCAGTCTATGAC-1 SI-GA-H1_BCB102 SI-GA-H1 BCB102 -0.292 0.515
## 4 1_AACTTCTCACGCTGAC-1 SI-GA-H1_BCB102 SI-GA-H1 BCB102 0.372 2.34
## 5 1_AAGTCGTGTGTTGCCG-1 SI-GA-H1_BCB102 SI-GA-H1 BCB102 -1.63 -0.236
## 6 1_AATGAAGCATCCAACA-1 SI-GA-H1_BCB102 SI-GA-H1 BCB102 0.822 2.90
## 7 1_ACAAAGAGTCGTACTA-1 SI-GA-H1_BCB102 SI-GA-H1 BCB102 3.28 3.97
## 8 1_ACACGCGCAGGTACGA-1 SI-GA-H1_BCB102 SI-GA-H1 BCB102 -3.65 -0.192
## 9 1_ACATGCATCACTTTGT-1 SI-GA-H1_BCB102 SI-GA-H1 BCB102 -0.273 4.09
## 10 1_ACGATCATCGACGAGA-1 SI-GA-H1_BCB102 SI-GA-H1 BCB102 -0.816 2.90
## # ℹ 3,570 more rowsPart 2 Signature visualisation
Data pre-processing
The object sce_obj we’ve been using was created as part
of a study on breast cancer systemic immune response. Peripheral blood
mononuclear cells have been sequenced for RNA at the single-cell level.
The steps used to generate the object are summarised below.
scran,scater, andDropletsUtilspackages have been used to eliminate empty droplets and dead cells. Samples were individually quality checked and cells were filtered for good gene coverage.Variable features were identified using
modelGeneVar.Read counts were scaled and normalised using logNormCounts from
scuttle.Data integration was performed using
fastMNNwith default parameters.PCA performed to reduce feature dimensionality.
Nearest-neighbor cell networks were calculated using 30 principal components.
2 UMAP dimensions were calculated using 30 principal components.
Cells with similar transcriptome profiles were grouped into clusters using Louvain clustering from
scran.
Analyse custom signature
The researcher analysing this dataset wanted to identify gamma delta T cells using a gene signature from a published paper (Pizzolato et al. 2019). We’ll show how that can be done here.
With tidySingleCellExperiment’s join_features we can
view the counts for genes in the signature as columns joined to our
single cell tibble.
sce_obj |>
join_features(c("CD3D", "TRDC", "TRGC1", "TRGC2", "CD8A", "CD8B"), shape = "wide")## # A SingleCellExperiment-tibble abstraction: 3,580 × 25
## # Features=482 | Cells=3580 | Assays=counts, logcounts
## .cell Barcode batch sample_id BCB S.Score G2M.Score Phase cell_type
## <chr> <fct> <fct> <chr> <fct> <dbl> <dbl> <fct> <fct>
## 1 1_AAATGGACA… AAATGG… 1 SI-GA-H1… BCB1… -2.07e-2 -0.0602 G1 TCR_V_De…
## 2 1_AACAAGAGT… AACAAG… 1 SI-GA-H1… BCB1… 2.09e-2 -0.0357 S CD8+_tra…
## 3 1_AACGTCAGT… AACGTC… 1 SI-GA-H1… BCB1… -2.54e-2 -0.133 G1 CD8+_hig…
## 4 1_AACTTCTCA… AACTTC… 1 SI-GA-H1… BCB1… 2.85e-2 -0.163 S CD8+_tra…
## 5 1_AAGTCGTGT… AAGTCG… 1 SI-GA-H1… BCB1… -2.30e-2 -0.0581 G1 MAIT
## 6 1_AATGAAGCA… AATGAA… 1 SI-GA-H1… BCB1… 1.09e-2 -0.0621 S CD4+_rib…
## 7 1_ACAAAGAGT… ACAAAG… 1 SI-GA-H1… BCB1… -2.06e-2 -0.0409 G1 CD4+_Tcm…
## 8 1_ACACGCGCA… ACACGC… 1 SI-GA-H1… BCB1… -3.95e-4 -0.176 G1 CD8+_hig…
## 9 1_ACATGCATC… ACATGC… 1 SI-GA-H1… BCB1… -4.09e-2 -0.0829 G1 CD4+_rib…
## 10 1_ACGATCATC… ACGATC… 1 SI-GA-H1… BCB1… 8.82e-2 -0.0397 S CD4+_rib…
## # ℹ 3,570 more rows
## # ℹ 16 more variables: nCount_RNA <int>, nFeature_RNA <int>, nCount_SCT <int>,
## # nFeature_SCT <int>, ident <fct>, file <chr>, sample <chr>, treatment <chr>,
## # CD3D <dbl>, TRDC <dbl>, TRGC1 <dbl>, TRGC2 <dbl>, CD8A <dbl>, CD8B <dbl>,
## # UMAP_1 <dbl>, UMAP_2 <dbl>We can use tidyverse mutate to create a column
containing the signature score. To generate the score, we scale the sum
of the 4 genes, CD3D, TRDC, TRGC1, TRGC2, and subtract the scaled sum of
the 2 genes, CD8A and CD8B. mutate is powerful in enabling
us to perform complex arithmetic operations easily.
sce_obj |>
join_features(c("CD3D", "TRDC", "TRGC1", "TRGC2", "CD8A", "CD8B"), shape = "wide") |>
mutate(
signature_score =
scales::rescale(CD3D + TRDC + TRGC1 + TRGC2, to = c(0, 1)) -
scales::rescale(CD8A + CD8B, to = c(0, 1))
) |>
select(.cell, signature_score, everything())## # A SingleCellExperiment-tibble abstraction: 3,580 × 26
## # Features=482 | Cells=3580 | Assays=counts, logcounts
## .cell signature_score Barcode batch sample_id BCB S.Score G2M.Score Phase
## <chr> <dbl> <fct> <fct> <chr> <fct> <dbl> <dbl> <fct>
## 1 1_AAA… 0.386 AAATGG… 1 SI-GA-H1… BCB1… -2.07e-2 -0.0602 G1
## 2 1_AAC… 0.108 AACAAG… 1 SI-GA-H1… BCB1… 2.09e-2 -0.0357 S
## 3 1_AAC… -0.789 AACGTC… 1 SI-GA-H1… BCB1… -2.54e-2 -0.133 G1
## 4 1_AAC… -0.0694 AACTTC… 1 SI-GA-H1… BCB1… 2.85e-2 -0.163 S
## 5 1_AAG… 0 AAGTCG… 1 SI-GA-H1… BCB1… -2.30e-2 -0.0581 G1
## 6 1_AAT… 0 AATGAA… 1 SI-GA-H1… BCB1… 1.09e-2 -0.0621 S
## 7 1_ACA… 0.108 ACAAAG… 1 SI-GA-H1… BCB1… -2.06e-2 -0.0409 G1
## 8 1_ACA… 0.170 ACACGC… 1 SI-GA-H1… BCB1… -3.95e-4 -0.176 G1
## 9 1_ACA… 0 ACATGC… 1 SI-GA-H1… BCB1… -4.09e-2 -0.0829 G1
## 10 1_ACG… 0.278 ACGATC… 1 SI-GA-H1… BCB1… 8.82e-2 -0.0397 S
## # ℹ 3,570 more rows
## # ℹ 17 more variables: cell_type <fct>, nCount_RNA <int>, nFeature_RNA <int>,
## # nCount_SCT <int>, nFeature_SCT <int>, ident <fct>, file <chr>,
## # sample <chr>, treatment <chr>, CD3D <dbl>, TRDC <dbl>, TRGC1 <dbl>,
## # TRGC2 <dbl>, CD8A <dbl>, CD8B <dbl>, UMAP_1 <dbl>, UMAP_2 <dbl>The gamma delta T cells could then be visualised by the signature score using Bioconductor’s visualisation functions.
sce_obj |>
join_features(
features = c("CD3D", "TRDC", "TRGC1", "TRGC2", "CD8A", "CD8B"), shape = "wide"
) |>
mutate(
signature_score =
scales::rescale(CD3D + TRDC + TRGC1 + TRGC2, to = c(0, 1)) -
scales::rescale(CD8A + CD8B, to = c(0, 1))
) |>
scater::plotUMAP(colour_by = "signature_score")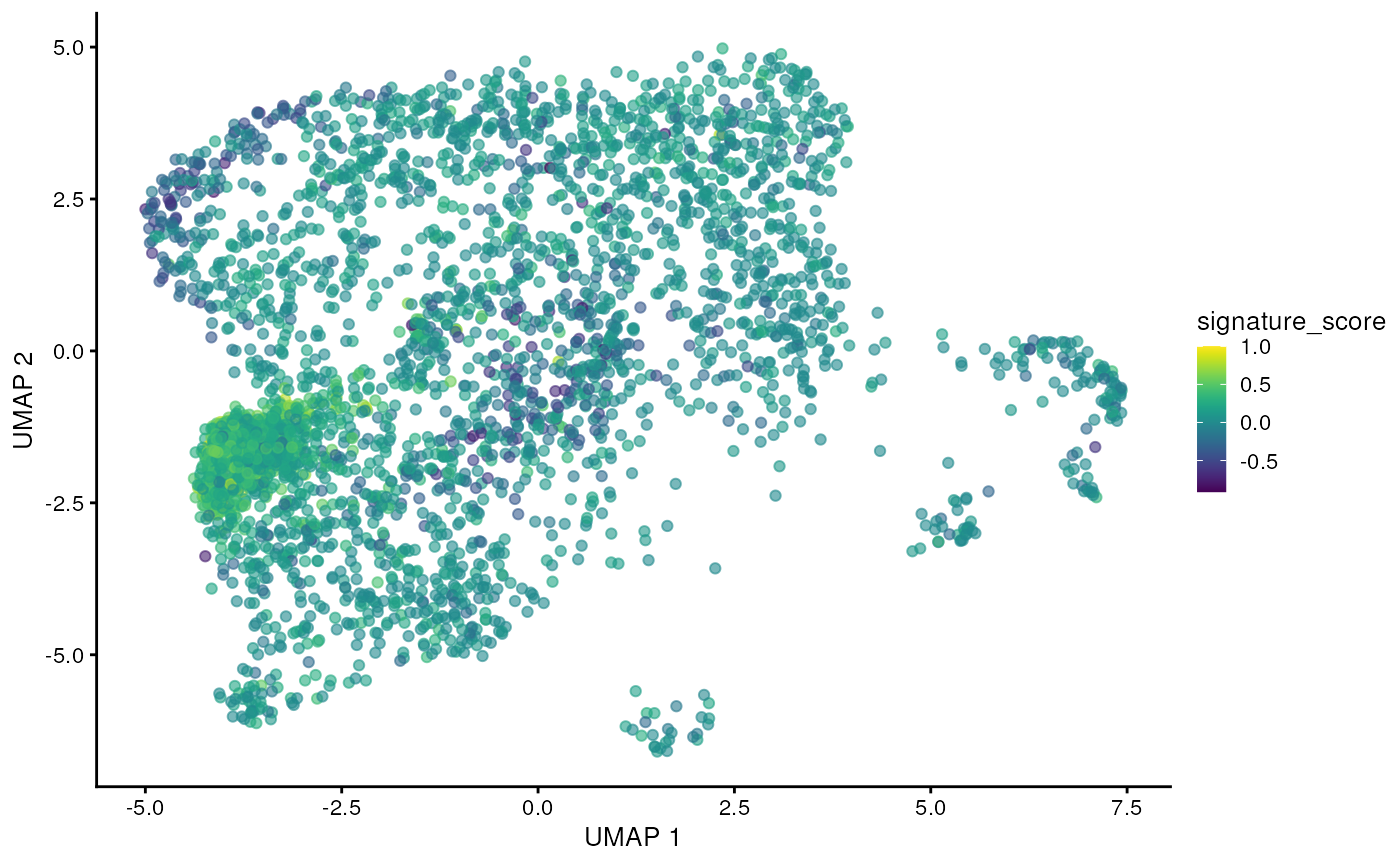
The cells could also be visualised using the popular and powerful
ggplot2 package, enabling the researcher to use ggplot
functions they were familiar with, and to customise the plot with great
flexibility.
sce_obj |>
join_features(
features = c("CD3D", "TRDC", "TRGC1", "TRGC2", "CD8A", "CD8B"), shape = "wide"
) |>
mutate(
signature_score =
scales::rescale(CD3D + TRDC + TRGC1 + TRGC2, to = c(0, 1)) -
scales::rescale(CD8A + CD8B, to = c(0, 1))
) |>
# plot cells with high score last so they're not obscured by other cells
arrange(signature_score) |>
ggplot(aes(UMAP_1, UMAP_2, color = signature_score)) +
geom_point() +
scale_color_distiller(palette = "Spectral") +
tidyomicsWorkshop::theme_multipanel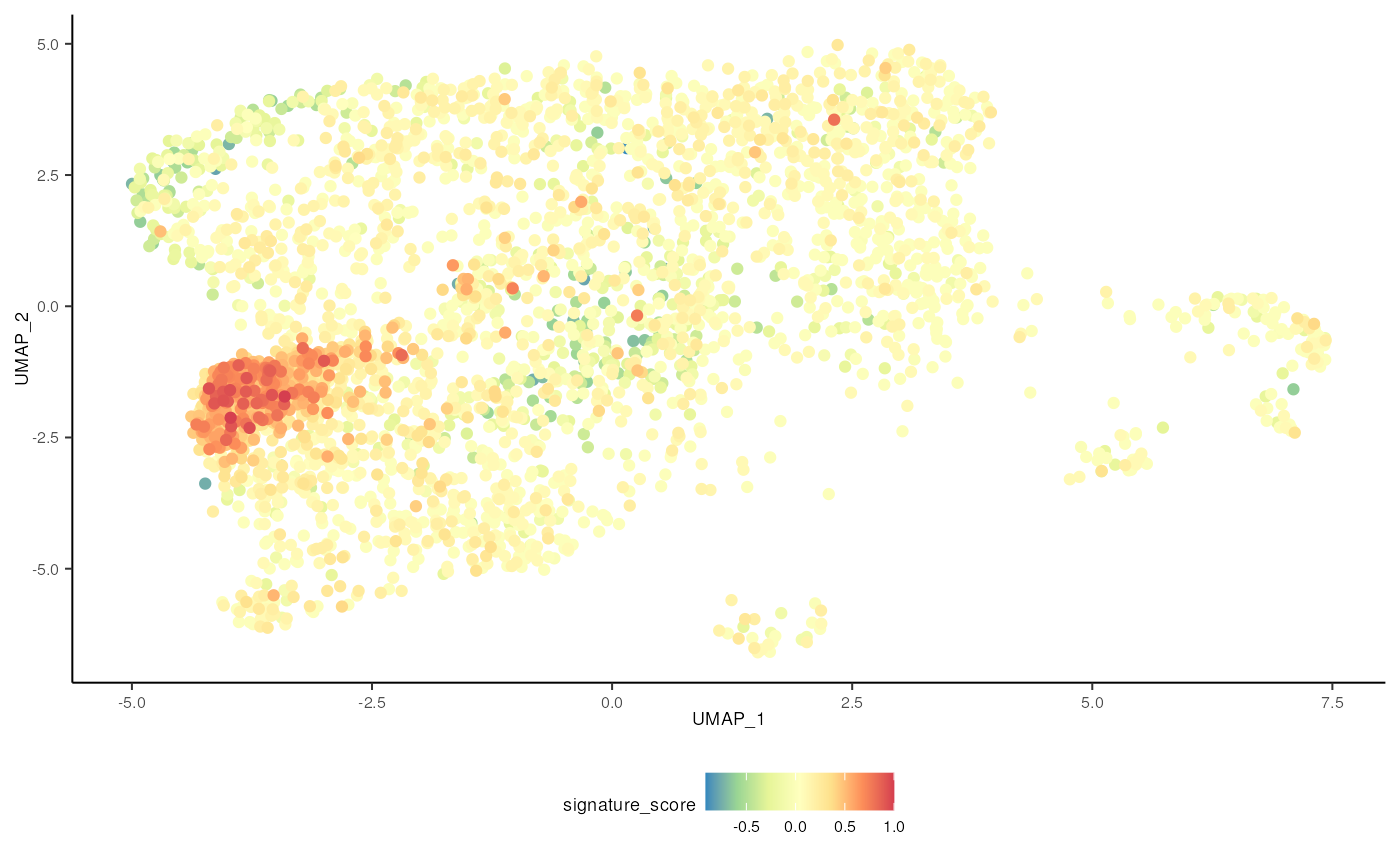
For exploratory analyses, we can select the gamma delta T cells, the red cluster on the left with high signature score. We’ll filter for cells with a signature score > 0.7.
sce_obj_gamma_delta <-
sce_obj |>
join_features(
features = c("CD3D", "TRDC", "TRGC1", "TRGC2", "CD8A", "CD8B"), shape = "wide"
) |>
mutate(
signature_score =
scales::rescale(CD3D + TRDC + TRGC1 + TRGC2, to = c(0, 1)) -
scales::rescale(CD8A + CD8B, to = c(0, 1))
) |>
# Proper cluster selection should be used instead (see supplementary material)
filter(signature_score > 0.7)For comparison, we show the alternative using base R and SingleCellExperiment. Note that the code contains more redundancy and intermediate objects.
counts_positive <-
assay(sce_obj, "logcounts")[c("CD3D", "TRDC", "TRGC1", "TRGC2"), ] |>
colSums() |>
scales::rescale(to = c(0, 1))
counts_negative <-
assay(sce_obj, "logcounts")[c("CD8A", "CD8B"), ] |>
colSums() |>
scales::rescale(to = c(0, 1))
sce_obj$signature_score <- counts_positive - counts_negative
sce_obj_gamma_delta <- sce_obj[, sce_obj$signature_score > 0.7]We can then focus on just these gamma delta T cells and chain Bioconductor and tidyverse commands together to analyse.
library(batchelor)
library(scater)
sce_obj_gamma_delta <-
sce_obj_gamma_delta |>
# Integrate - using batchelor.
multiBatchNorm(batch = colData(sce_obj_gamma_delta)$sample) |>
fastMNN(batch = colData(sce_obj_gamma_delta)$sample) |>
# Join metadata removed by fastMNN - using tidyverse
left_join(as_tibble(sce_obj_gamma_delta)) |>
# Dimension reduction - using scater
runUMAP(ncomponents = 2, dimred = "corrected")Visualise gamma delta T cells. As we have used rough threshold we are left with only few cells. Proper cluster selection should be used instead (see supplementary material).
sce_obj_gamma_delta |> plotUMAP() 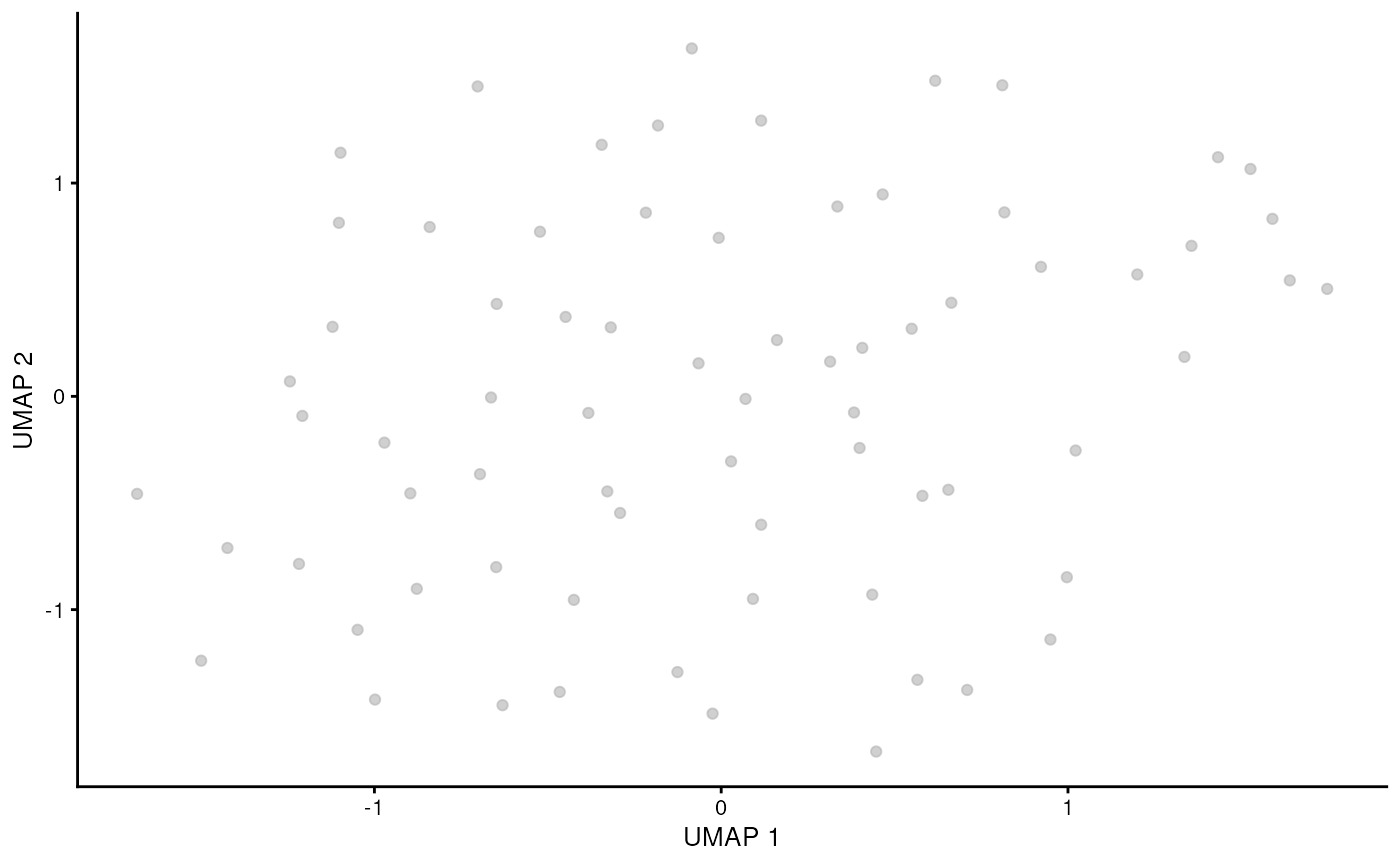
It is also possible to visualise the cells as a 3D plot using plotly. The example data used here only contains a few genes, for the sake of time and size in this demonstration, but below is how you could generate the 3 dimensions needed for 3D plot with a full dataset.
single_cell_object |>
RunUMAP(dims = 1:30, n.components = 3L, spread = 0.5, min.dist = 0.01, n.neighbors = 10L)We’ll demonstrate creating a 3D plot using some data that has 3 UMAP dimensions. This is a fantastic way to visualise both reduced dimensions and metadata in the same representation.
pbmc <- tidyomicsWorkshop::sce_obj_UMAP3
pbmc |>
plot_ly(
x = ~`UMAP_1`,
y = ~`UMAP_2`,
z = ~`UMAP_3`,
color = ~cell_type,
colors = dittoSeq::dittoColors()
) %>%
add_markers(size = I(1))Part 3 Nested analyses
When analysing single cell data is sometimes necessary to perform calculations on data subsets. For example, we might want to estimate difference in mRNA abundance between two condition for each cell type.
tidyr and purrr offer a great tool to
perform iterativre analyses in a functional way.
We use tidyverse nest to group the data. The command
below will create a tibble containing a column with a
SummarizedExperiment object for each cell type. nest is
similar to tidyverse group_by, except with
nest each group is stored in a single row, and can be a
complex object such as SingleCellExperiment.
Let’s import some libraries
##
## Attaching package: 'purrr'## The following object is masked from 'package:GenomicRanges':
##
## reduce## The following object is masked from 'package:IRanges':
##
## reduceFirst let’s have a look to the cell types that constitute this dataset
sce_obj |>
count(cell_type)## tidySingleCellExperiment says: A data frame is returned for independent data analysis.## # A tibble: 13 × 2
## cell_type n
## <fct> <int>
## 1 CD4+_ribosome_rich 299
## 2 CD4+_Tcm_S100A4_IL32_IL7R_VIM 407
## 3 CD8+_high_ribonucleosome 404
## 4 CD8+_Tcm 277
## 5 CD8+_transitional_effector_GZMK_KLRB1_LYAR4 343
## 6 HSC_lymphoid_myeloid_like 116
## 7 limphoid_myeloid_like_HLA_CD74 26
## 8 MAIT 164
## 9 NK_cells 337
## 10 NK_cycling 28
## 11 T_cell:CD8+_other 173
## 12 TCR_V_Delta_1 651
## 13 TCR_V_Delta_2 355Let’s group the cells based on cell identity using
nest
sce_obj_nested =
sce_obj |>
nest(sce = -cell_type)
sce_obj_nested## # A tibble: 13 × 2
## cell_type sce
## <fct> <list>
## 1 TCR_V_Delta_2 <SnglCllE[,355]>
## 2 CD8+_transitional_effector_GZMK_KLRB1_LYAR4 <SnglCllE[,343]>
## 3 CD8+_high_ribonucleosome <SnglCllE[,404]>
## 4 MAIT <SnglCllE[,164]>
## 5 CD4+_ribosome_rich <SnglCllE[,299]>
## 6 CD4+_Tcm_S100A4_IL32_IL7R_VIM <SnglCllE[,407]>
## 7 NK_cells <SnglCllE[,337]>
## 8 TCR_V_Delta_1 <SnglCllE[,651]>
## 9 HSC_lymphoid_myeloid_like <SnglCllE[,116]>
## 10 T_cell:CD8+_other <SnglCllE[,173]>
## 11 NK_cycling <SnglCllE[,28]>
## 12 CD8+_Tcm <SnglCllE[,277]>
## 13 limphoid_myeloid_like_HLA_CD74 <SnglCllE[,26]>Let’s see what the first element of the Surat column looks like
## [[1]]
## # A SingleCellExperiment-tibble abstraction: 355 × 18
## # Features=482 | Cells=355 | Assays=counts, logcounts
## .cell Barcode batch sample_id BCB S.Score G2M.Score Phase nCount_RNA
## <chr> <fct> <fct> <chr> <fct> <dbl> <dbl> <fct> <int>
## 1 1_AAATGGAC… AAATGG… 1 SI-GA-H1… BCB1… -0.0207 -0.0602 G1 215
## 2 1_ATCACGAG… ATCACG… 1 SI-GA-H1… BCB1… 0.0103 -0.109 S 271
## 3 1_ATCGCCTC… ATCGCC… 1 SI-GA-H1… BCB1… -0.0241 -0.196 G1 640
## 4 1_CGATGGCT… CGATGG… 1 SI-GA-H1… BCB1… -0.0628 -0.213 G1 495
## 5 1_CGTGCTTG… CGTGCT… 1 SI-GA-H1… BCB1… -0.0403 -0.0371 G1 158
## 6 1_CTTGAGAA… CTTGAG… 1 SI-GA-H1… BCB1… 0.00494 -0.0548 S 173
## 7 1_GAGTGTTA… GAGTGT… 1 SI-GA-H1… BCB1… 0.0109 -0.0760 S 187
## 8 1_GTACAGTG… GTACAG… 1 SI-GA-H1… BCB1… 0.0265 -0.0922 S 186
## 9 1_TTCCTTCT… TTCCTT… 1 SI-GA-H1… BCB1… -0.00800 -0.0796 G1 1881
## 10 2_AAGACTCA… AAGACT… 1 SI-GA-H3… BCB1… -0.0350 -0.0878 G1 241
## # ℹ 345 more rows
## # ℹ 9 more variables: nFeature_RNA <int>, nCount_SCT <int>, nFeature_SCT <int>,
## # ident <fct>, file <chr>, sample <chr>, treatment <chr>, UMAP_1 <dbl>,
## # UMAP_2 <dbl>Now, let’s perform a differential gene-transcript abundance analysis between the two conditions for each cell type.
sce_obj_nested =
sce_obj_nested |>
# Filter for sample with more than 30 cells
mutate(sce = map(sce, ~ .x |> add_count(sample) |> filter(n > 50))) |>
filter(map_int(sce, ncol) > 0) |>
# Select significant genes
mutate(sce = imap(
sce,
~ .x %>% {print(.y); (.)} |>
# Integrate - using batchelor.
multiBatchNorm(batch = colData(.x)$sample) |>
fastMNN(batch = colData(.x)$sample) |>
# Join metadata removed by fastMNN - using tidyverse
left_join(as_tibble(.x)) |>
# Dimension reduction - using scater
runUMAP(ncomponents = 2, dimred = "corrected")
))## [1] 1
## [1] 2
## [1] 3
## [1] 4
## [1] 5
## [1] 6
## [1] 7
## [1] 8
## [1] 9## Warning: There were 2 warnings in `mutate()`.
## The first warning was:
## ℹ In argument: `sce = imap(...)`.
## Caused by warning:
## ! You're computing too large a percentage of total singular values, use a standard svd instead.
## ℹ Run `dplyr::last_dplyr_warnings()` to see the 1 remaining warning.
sce_obj_nested## # A tibble: 9 × 2
## cell_type sce
## <fct> <list>
## 1 TCR_V_Delta_2 <SnglCllE[,234]>
## 2 CD8+_transitional_effector_GZMK_KLRB1_LYAR4 <SnglCllE[,177]>
## 3 CD8+_high_ribonucleosome <SnglCllE[,218]>
## 4 CD4+_ribosome_rich <SnglCllE[,57]>
## 5 CD4+_Tcm_S100A4_IL32_IL7R_VIM <SnglCllE[,211]>
## 6 NK_cells <SnglCllE[,170]>
## 7 TCR_V_Delta_1 <SnglCllE[,478]>
## 8 HSC_lymphoid_myeloid_like <SnglCllE[,89]>
## 9 CD8+_Tcm <SnglCllE[,269]>We can the lies the top genes with the heat map iteratively across the cell types
## # A tibble: 9 × 3
## cell_type sce umap
## <fct> <list> <list>
## 1 TCR_V_Delta_2 <SnglCllE[,234]> <gg>
## 2 CD8+_transitional_effector_GZMK_KLRB1_LYAR4 <SnglCllE[,177]> <gg>
## 3 CD8+_high_ribonucleosome <SnglCllE[,218]> <gg>
## 4 CD4+_ribosome_rich <SnglCllE[,57]> <gg>
## 5 CD4+_Tcm_S100A4_IL32_IL7R_VIM <SnglCllE[,211]> <gg>
## 6 NK_cells <SnglCllE[,170]> <gg>
## 7 TCR_V_Delta_1 <SnglCllE[,478]> <gg>
## 8 HSC_lymphoid_myeloid_like <SnglCllE[,89]> <gg>
## 9 CD8+_Tcm <SnglCllE[,269]> <gg>Let’s have a look to the first heatmap
## [[1]]
You can do this whole analysis without saving any temporary variable using the piping functionality of tidy R programming
sce_obj |>
# Nest
nest(sce = -cell_type) |>
# Filter for sample with more than 30 cells
mutate(sce = map(sce, ~ .x |> add_count(sample) |> filter(n > 50))) |>
filter(map_int(sce, ncol) > 0) |>
# Select significant genes
mutate(sce = imap(
sce,
~ .x %>% {print(.y); (.)} |>
# Integrate - using batchelor.
multiBatchNorm(batch = colData(.x)$sample) |>
fastMNN(batch = colData(.x)$sample) |>
# Join metadata removed by fastMNN - using tidyverse
left_join(as_tibble(.x)) |>
# Dimension reduction - using scater
runUMAP(ncomponents = 2, dimred = "corrected")
)) |>
# Build heatmaps
mutate(umap = map(sce,plotUMAP)) |>
# Extract heatmaps
pull(umap)Exercises
- Let’s suppose that you want to perform the analyses only for cell types that have a total number of cells bigger than 1000. For example, if a cell type has less than a sum of 1000 cells across all samples, that cell type will be dropped from the dataset.
- Answer this question avoiding to save temporary variables, and using the function add_count to count the cells (before nesting), and then filter
- Answer this question avoiding to save temporary variables, and using the function map_int to count the cells (after nesting), and the filter
Session Information
## R version 4.3.1 (2023-06-16)
## Platform: x86_64-pc-linux-gnu (64-bit)
## Running under: Ubuntu 22.04.2 LTS
##
## Matrix products: default
## BLAS: /usr/lib/x86_64-linux-gnu/openblas-pthread/libblas.so.3
## LAPACK: /usr/lib/x86_64-linux-gnu/openblas-pthread/libopenblasp-r0.3.20.so; LAPACK version 3.10.0
##
## locale:
## [1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
## [3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
## [5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
## [7] LC_PAPER=en_US.UTF-8 LC_NAME=C
## [9] LC_ADDRESS=C LC_TELEPHONE=C
## [11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
##
## time zone: UTC
## tzcode source: system (glibc)
##
## attached base packages:
## [1] stats4 stats graphics grDevices utils datasets methods
## [8] base
##
## other attached packages:
## [1] purrr_1.0.1 scater_1.28.0
## [3] scuttle_1.10.1 batchelor_1.16.0
## [5] tidySingleCellExperiment_1.10.0 ttservice_0.3.6
## [7] dittoSeq_1.12.0 colorspace_2.1-0
## [9] dplyr_1.1.2 plotly_4.10.2
## [11] ggplot2_3.4.2 SingleCellExperiment_1.22.0
## [13] SummarizedExperiment_1.30.2 Biobase_2.60.0
## [15] GenomicRanges_1.52.0 GenomeInfoDb_1.36.1
## [17] IRanges_2.34.1 S4Vectors_0.38.1
## [19] BiocGenerics_0.46.0 MatrixGenerics_1.12.2
## [21] matrixStats_1.0.0
##
## loaded via a namespace (and not attached):
## [1] bitops_1.0-7 gridExtra_2.3
## [3] rlang_1.1.1 magrittr_2.0.3
## [5] ggridges_0.5.4 compiler_4.3.1
## [7] DelayedMatrixStats_1.22.1 systemfonts_1.0.4
## [9] vctrs_0.6.3 stringr_1.5.0
## [11] pkgconfig_2.0.3 crayon_1.5.2
## [13] fastmap_1.1.1 XVector_0.40.0
## [15] ellipsis_0.3.2 labeling_0.4.2
## [17] utf8_1.2.3 rmarkdown_2.23
## [19] ggbeeswarm_0.7.2 ragg_1.2.5
## [21] xfun_0.39 zlibbioc_1.46.0
## [23] cachem_1.0.8 beachmat_2.16.0
## [25] jsonlite_1.8.7 highr_0.10
## [27] DelayedArray_0.26.6 BiocParallel_1.34.2
## [29] irlba_2.3.5.1 parallel_4.3.1
## [31] R6_2.5.1 bslib_0.5.0
## [33] stringi_1.7.12 RColorBrewer_1.1-3
## [35] jquerylib_0.1.4 Rcpp_1.0.10
## [37] knitr_1.43 FNN_1.1.3.2
## [39] igraph_1.5.0 Matrix_1.5-4.1
## [41] tidyselect_1.2.0 viridis_0.6.3
## [43] yaml_2.3.7 codetools_0.2-19
## [45] tidyomicsWorkshop_0.16.2 lattice_0.21-8
## [47] tibble_3.2.1 withr_2.5.0
## [49] evaluate_0.21 desc_1.4.2
## [51] pillar_1.9.0 generics_0.1.3
## [53] rprojroot_2.0.3 RCurl_1.98-1.12
## [55] sparseMatrixStats_1.12.2 munsell_0.5.0
## [57] scales_1.2.1 glue_1.6.2
## [59] pheatmap_1.0.12 lazyeval_0.2.2
## [61] tools_4.3.1 BiocNeighbors_1.18.0
## [63] data.table_1.14.8 ScaledMatrix_1.8.1
## [65] fs_1.6.2 cowplot_1.1.1
## [67] grid_4.3.1 tidyr_1.3.0
## [69] crosstalk_1.2.0 GenomeInfoDbData_1.2.10
## [71] beeswarm_0.4.0 BiocSingular_1.16.0
## [73] vipor_0.4.5 cli_3.6.1
## [75] rsvd_1.0.5 textshaping_0.3.6
## [77] fansi_1.0.4 S4Arrays_1.0.4
## [79] viridisLite_0.4.2 uwot_0.1.16
## [81] ResidualMatrix_1.10.0 gtable_0.3.3
## [83] sass_0.4.6 digest_0.6.32
## [85] ggrepel_0.9.3 farver_2.1.1
## [87] htmlwidgets_1.6.2 memoise_2.0.1
## [89] htmltools_0.5.5 pkgdown_2.0.7
## [91] lifecycle_1.0.3 httr_1.4.6References