Getting started with the plyranges package
Stuart Lee
2025-11-20
Source:vignettes/an-introduction.Rmd
an-introduction.Rmd
Ranges revisited
In Bioconductor there are two classes, IRanges and
GRanges, that are standard data structures for representing
genomics data. Throughout this document I refer to either of these
classes as Ranges if an operation can be performed on
either class, otherwise I explicitly mention if a function is
appropriate for an IRanges or GRanges.
Ranges objects can either represent sets of integers as
IRanges (which have start, end and width attributes) or
represent genomic intervals (which have additional attributes, sequence
name, and strand) as GRanges. In addition, both types of
Ranges can store information about their intervals as
metadata columns (for example GC content over a genomic interval).
Ranges objects follow the tidy data principle: each row
of a Ranges object corresponds to an interval, while each
column will represent a variable about that interval, and generally each
object will represent a single unit of observation (like gene
annotations).
Consequently, Ranges objects provide a powerful
representation for reasoning about genomic data. In this vignette, you
will learn more about Ranges objects and how via grouping,
restriction and summarisation you can perform common data tasks.
Constructing Ranges
To construct an IRanges we require that there are at
least two columns that represent at either a starting coordinate,
finishing coordinate or the width of the interval.
suppressPackageStartupMessages(library(plyranges))
set.seed(100)
df <- data.frame(start=c(2:-1, 13:15),
width=c(0:3, 2:0))
# produces IRanges
rng <- df %>% as_iranges()
rng## IRanges object with 7 ranges and 0 metadata columns:
## start end width
## <integer> <integer> <integer>
## [1] 2 1 0
## [2] 1 1 1
## [3] 0 1 2
## [4] -1 1 3
## [5] 13 14 2
## [6] 14 14 1
## [7] 15 14 0To construct a GRanges we require a column that
represents that sequence name ( contig or chromosome id), and an
optional column to represent the strandedness of an interval.
# seqname is required for GRanges, metadata is automatically kept
grng <- df %>%
transform(seqnames = sample(c("chr1", "chr2"), 7, replace = TRUE),
strand = sample(c("+", "-"), 7, replace = TRUE),
gc = runif(7)) %>%
as_granges()
grng## GRanges object with 7 ranges and 1 metadata column:
## seqnames ranges strand | gc
## <Rle> <IRanges> <Rle> | <numeric>
## [1] chr2 2-1 - | 0.762551
## [2] chr1 1 - | 0.669022
## [3] chr2 0-1 + | 0.204612
## [4] chr2 -1-1 - | 0.357525
## [5] chr1 13-14 - | 0.359475
## [6] chr1 14 - | 0.690291
## [7] chr2 15-14 - | 0.535811
## -------
## seqinfo: 2 sequences from an unspecified genome; no seqlengthsArithmetic on Ranges
Sometimes you want to modify a genomic interval by altering the width
of the interval while leaving the start, end or midpoint of the
coordinates unaltered. This is achieved with the mutate
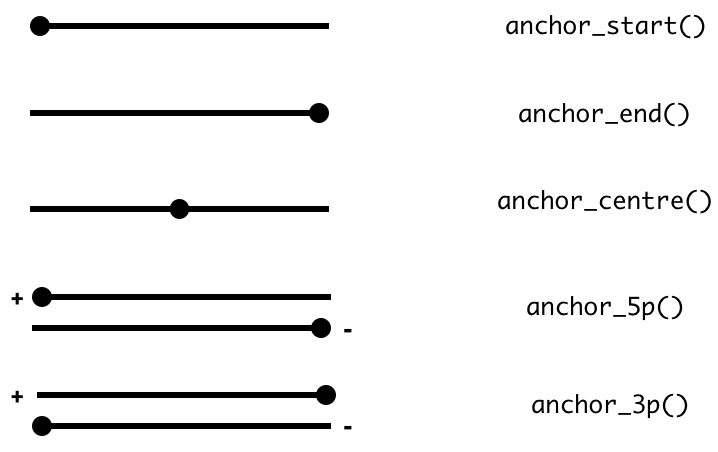
verb along with anchor_* adverbs.
The act of anchoring fixes either the start, end, center coordinates
of the Range object, as shown in the figure and code below
and anchors are used in combination with either mutate or
stretch. By default, the start coordinate will be anchored,
so regardless of strand. For behavior similar to
GenomicRanges::resize, use anchor_5p.
rng <- as_iranges(data.frame(start=c(1, 2, 3), end=c(5, 2, 8)))
grng <- as_granges(data.frame(start=c(1, 2, 3), end=c(5, 2, 8),
seqnames = "seq1",
strand = c("+", "*", "-")))
mutate(rng, width = 10)## IRanges object with 3 ranges and 0 metadata columns:
## start end width
## <integer> <integer> <integer>
## [1] 1 10 10
## [2] 2 11 10
## [3] 3 12 10
mutate(anchor_start(rng), width = 10)## IRanges object with 3 ranges and 0 metadata columns:
## start end width
## <integer> <integer> <integer>
## [1] 1 10 10
## [2] 2 11 10
## [3] 3 12 10
mutate(anchor_end(rng), width = 10)## IRanges object with 3 ranges and 0 metadata columns:
## start end width
## <integer> <integer> <integer>
## [1] -4 5 10
## [2] -7 2 10
## [3] -1 8 10
mutate(anchor_center(rng), width = 10)## IRanges object with 3 ranges and 0 metadata columns:
## start end width
## <integer> <integer> <integer>
## [1] -2 7 10
## [2] -3 6 10
## [3] 1 10 10## GRanges object with 3 ranges and 0 metadata columns:
## seqnames ranges strand
## <Rle> <IRanges> <Rle>
## [1] seq1 -4-5 +
## [2] seq1 -7-2 *
## [3] seq1 3-12 -
## -------
## seqinfo: 1 sequence from an unspecified genome; no seqlengths## GRanges object with 3 ranges and 0 metadata columns:
## seqnames ranges strand
## <Rle> <IRanges> <Rle>
## [1] seq1 1-10 +
## [2] seq1 2-11 *
## [3] seq1 -1-8 -
## -------
## seqinfo: 1 sequence from an unspecified genome; no seqlengthsSimilarly, you can modify the width of an interval using the
stretch verb. Without anchoring, this function will extend
the interval in either direction by an integer amount. With anchoring,
either the start, end or midpoint are preserved.
rng2 <- stretch(anchor_center(rng), 10)
rng2## IRanges object with 3 ranges and 0 metadata columns:
## start end width
## <integer> <integer> <integer>
## [1] -4 10 15
## [2] -3 7 11
## [3] -2 13 16
stretch(anchor_end(rng2), 10)## IRanges object with 3 ranges and 0 metadata columns:
## start end width
## <integer> <integer> <integer>
## [1] -14 10 25
## [2] -13 7 21
## [3] -12 13 26
stretch(anchor_start(rng2), 10)## IRanges object with 3 ranges and 0 metadata columns:
## start end width
## <integer> <integer> <integer>
## [1] -4 20 25
## [2] -3 17 21
## [3] -2 23 26## GRanges object with 3 ranges and 0 metadata columns:
## seqnames ranges strand
## <Rle> <IRanges> <Rle>
## [1] seq1 -9-5 +
## [2] seq1 -8-2 *
## [3] seq1 3-18 -
## -------
## seqinfo: 1 sequence from an unspecified genome; no seqlengths## GRanges object with 3 ranges and 0 metadata columns:
## seqnames ranges strand
## <Rle> <IRanges> <Rle>
## [1] seq1 1-15 +
## [2] seq1 2-12 *
## [3] seq1 -7-8 -
## -------
## seqinfo: 1 sequence from an unspecified genome; no seqlengthsRanges can be shifted left or right. If strand
information is available we can also shift upstream or downstream.
shift_left(rng, 100)## IRanges object with 3 ranges and 0 metadata columns:
## start end width
## <integer> <integer> <integer>
## [1] -99 -95 5
## [2] -98 -98 1
## [3] -97 -92 6
shift_right(rng, 100)## IRanges object with 3 ranges and 0 metadata columns:
## start end width
## <integer> <integer> <integer>
## [1] 101 105 5
## [2] 102 102 1
## [3] 103 108 6
shift_upstream(grng, 100)## GRanges object with 3 ranges and 0 metadata columns:
## seqnames ranges strand
## <Rle> <IRanges> <Rle>
## [1] seq1 -99--95 +
## [2] seq1 -98 *
## [3] seq1 103-108 -
## -------
## seqinfo: 1 sequence from an unspecified genome; no seqlengths
shift_downstream(grng, 100)## GRanges object with 3 ranges and 0 metadata columns:
## seqnames ranges strand
## <Rle> <IRanges> <Rle>
## [1] seq1 101-105 +
## [2] seq1 102 *
## [3] seq1 -97--92 -
## -------
## seqinfo: 1 sequence from an unspecified genome; no seqlengthsGrouping Ranges
plyranges introduces a new class of Ranges
called RangesGrouped, this is a similar idea to the grouped
data.frame\tibble in dplyr.
Grouping can act on either the core components or the metadata
columns of a Ranges object.
It is most effective when combined with other verbs such as
mutate(), summarise(), filter(),
reduce_ranges() or disjoin_ranges().
grng <- data.frame(seqnames = sample(c("chr1", "chr2"), 7, replace = TRUE),
strand = sample(c("+", "-"), 7, replace = TRUE),
gc = runif(7),
start = 1:7,
width = 10) %>%
as_granges()
grng_by_strand <- grng %>%
group_by(strand)
grng_by_strand## GRanges object with 7 ranges and 1 metadata column:
## Groups: strand [2]
## seqnames ranges strand | gc
## <Rle> <IRanges> <Rle> | <numeric>
## [1] chr2 1-10 - | 0.889454
## [2] chr2 2-11 + | 0.180407
## [3] chr1 3-12 - | 0.629391
## [4] chr2 4-13 + | 0.989564
## [5] chr1 5-14 - | 0.130289
## [6] chr1 6-15 - | 0.330661
## [7] chr2 7-16 - | 0.865121
## -------
## seqinfo: 2 sequences from an unspecified genome; no seqlengthsRestricting Ranges
The verb filter can be used to restrict rows in the
Ranges. Note that grouping will cause the
filter to act within each group of the data.
## GRanges object with 2 ranges and 1 metadata column:
## seqnames ranges strand | gc
## <Rle> <IRanges> <Rle> | <numeric>
## [1] chr2 2-11 + | 0.180407
## [2] chr1 5-14 - | 0.130289
## -------
## seqinfo: 2 sequences from an unspecified genome; no seqlengths## GRanges object with 2 ranges and 1 metadata column:
## Groups: strand [2]
## seqnames ranges strand | gc
## <Rle> <IRanges> <Rle> | <numeric>
## [1] chr2 1-10 - | 0.889454
## [2] chr2 4-13 + | 0.989564
## -------
## seqinfo: 2 sequences from an unspecified genome; no seqlengthsWe also provide the convenience methods
filter_by_overlaps and filter_by_non_overlaps
for restricting by any overlapping Ranges.
ir0 <- data.frame(start = c(5,10, 15,20), width = 5) %>%
as_iranges()
ir1 <- data.frame(start = 2:6, width = 3:7) %>%
as_iranges()
ir0## IRanges object with 4 ranges and 0 metadata columns:
## start end width
## <integer> <integer> <integer>
## [1] 5 9 5
## [2] 10 14 5
## [3] 15 19 5
## [4] 20 24 5
ir1## IRanges object with 5 ranges and 0 metadata columns:
## start end width
## <integer> <integer> <integer>
## [1] 2 4 3
## [2] 3 6 4
## [3] 4 8 5
## [4] 5 10 6
## [5] 6 12 7
ir0 %>% filter_by_overlaps(ir1)## IRanges object with 2 ranges and 0 metadata columns:
## start end width
## <integer> <integer> <integer>
## [1] 5 9 5
## [2] 10 14 5
ir0 %>% filter_by_non_overlaps(ir1)## IRanges object with 2 ranges and 0 metadata columns:
## start end width
## <integer> <integer> <integer>
## [1] 15 19 5
## [2] 20 24 5Summarising Ranges
The summarise function will return a
DataFrame because the information required to return a
Ranges object is lost. It is often most useful to use
summarise() in combination with the group_by()
family of functions.
ir1 <- ir1 %>%
mutate(gc = runif(length(.)))
ir0 %>%
group_by_overlaps(ir1) %>%
summarise(gc = mean(gc))## DataFrame with 2 rows and 2 columns
## query gc
## <integer> <numeric>
## 1 1 0.675555
## 2 2 0.635795Joins, or another way at looking at overlaps between
Ranges
A join acts on two GRanges objects, a query and a subject.
query <- data.frame(seqnames = "chr1",
strand = c("+", "-"),
start = c(1, 9),
end = c(7, 10),
key.a = letters[1:2]) %>%
as_granges()
subject <- data.frame(seqnames = "chr1",
strand = c("-", "+"),
start = c(2, 6),
end = c(4, 8),
key.b = LETTERS[1:2]) %>%
as_granges()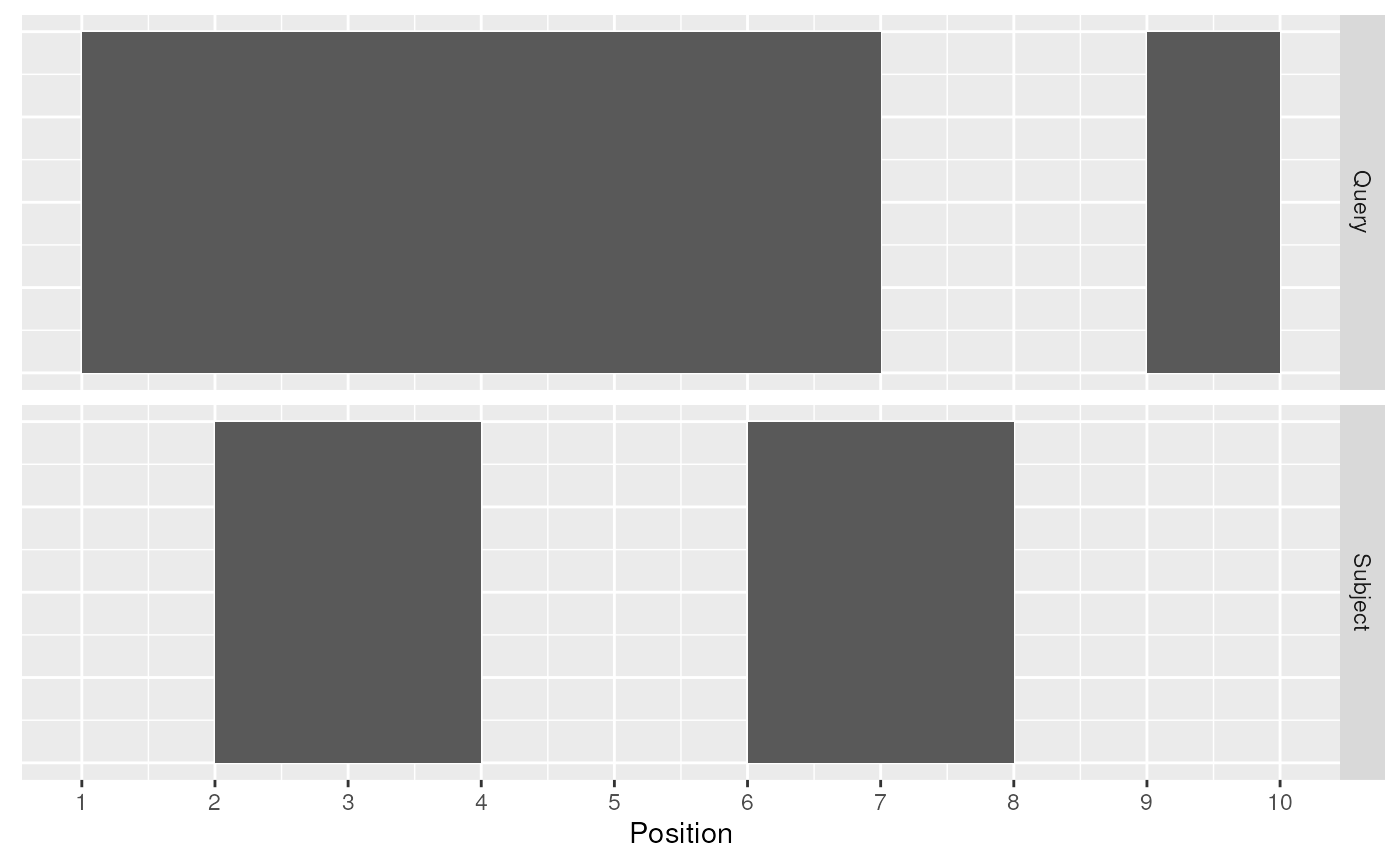
Query and Subject Ranges
The join operator is relational in the sense that metadata from the
query and subject ranges is retained in the joined range. All join
operators in the plyranges DSL generate a set of hits based
on overlap or proximity of ranges and use those hits to merge the two
datasets in different ways. There are four supported matching
algorithms: overlap, nearest, precede, and
follow. We can further restrict the matching by whether the
query is completely within the subject, and adding the
directed suffix ensures that matching ranges have the same
direction (strand).
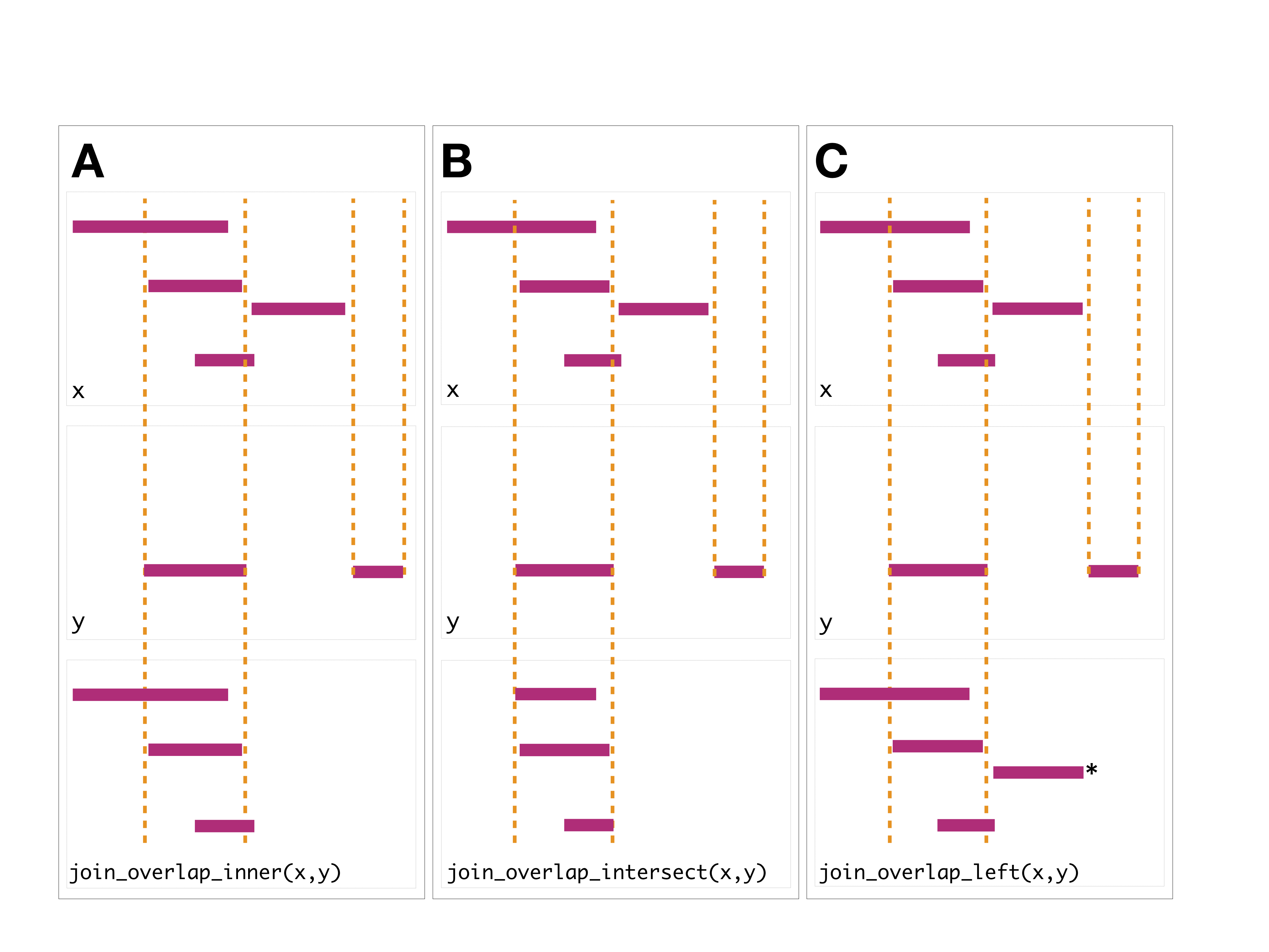
The first function, join_overlap_intersect() will return
a Ranges object where the start, end, and width coordinates
correspond to the amount of any overlap between the left and right input
Ranges. It also returns any metadatain the subject range if
the subject overlaps the query.
intersect_rng <- join_overlap_intersect(query, subject)
intersect_rng## GRanges object with 2 ranges and 2 metadata columns:
## seqnames ranges strand | key.a key.b
## <Rle> <IRanges> <Rle> | <character> <character>
## [1] chr1 2-4 + | a A
## [2] chr1 6-7 + | a B
## -------
## seqinfo: 1 sequence from an unspecified genome; no seqlengths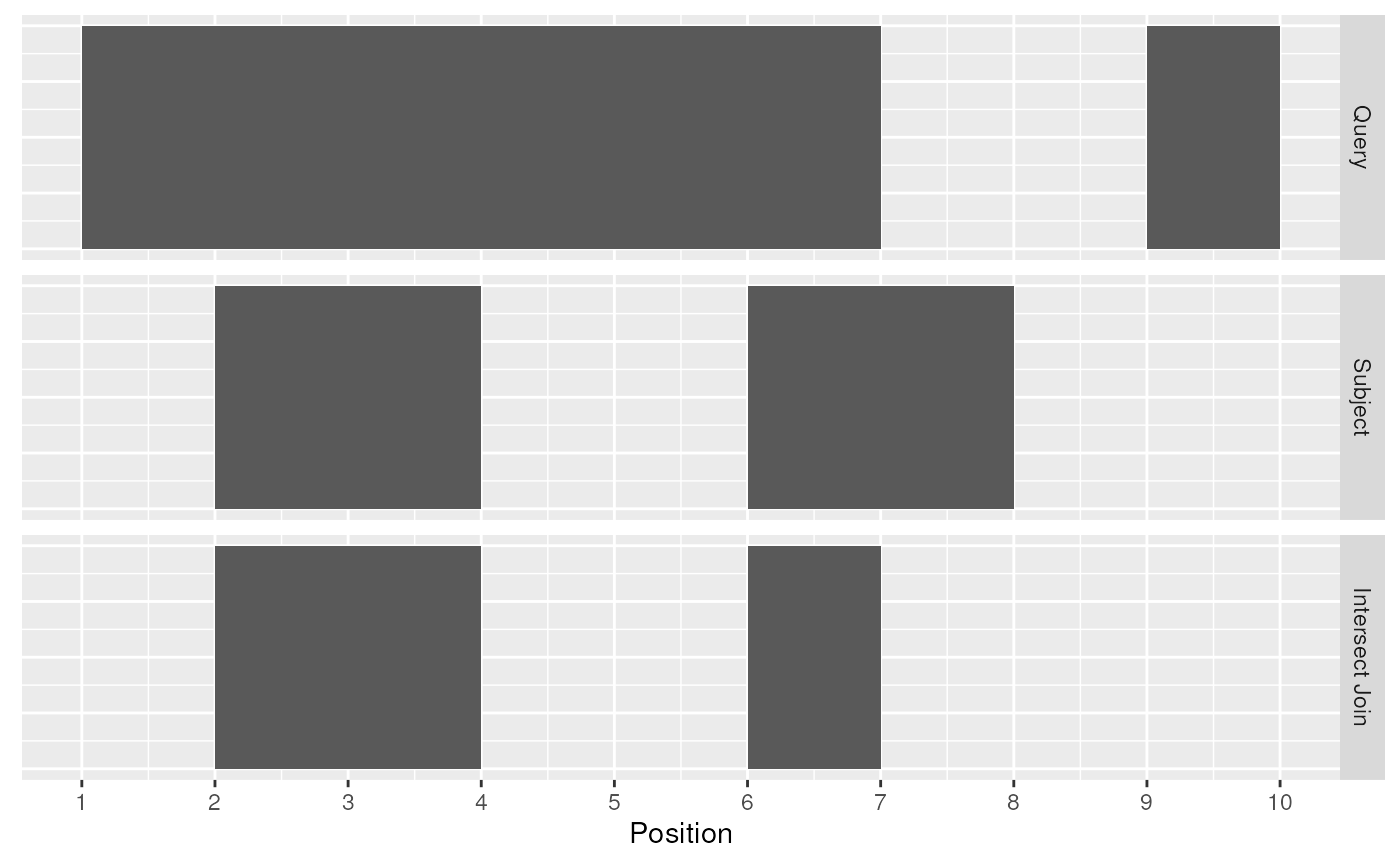
Intersect Join
The join_overlap_inner() function will return the
Ranges in the query that overlap any Ranges in
the subject. Like the join_overlap_intersect() function
metadata of the subject Range is returned if it overlaps
the query.
inner_rng <- join_overlap_inner(query, subject)
inner_rng## GRanges object with 2 ranges and 2 metadata columns:
## seqnames ranges strand | key.a key.b
## <Rle> <IRanges> <Rle> | <character> <character>
## [1] chr1 1-7 + | a A
## [2] chr1 1-7 + | a B
## -------
## seqinfo: 1 sequence from an unspecified genome; no seqlengths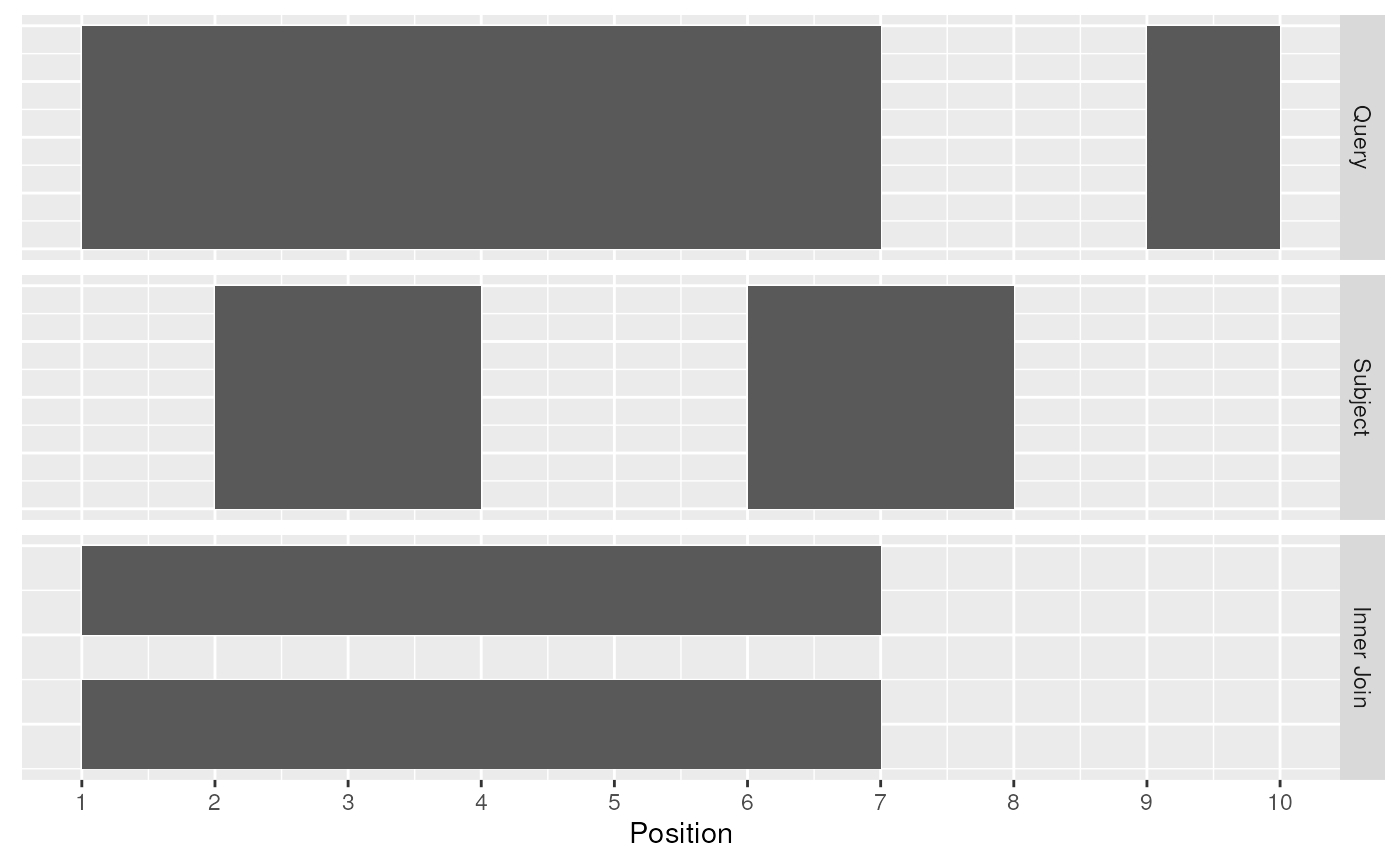
Inner Join
We also provide a convenience method called
find_overlaps that computes the same result as
join_overlap_inner().
find_overlaps(query, subject)## GRanges object with 2 ranges and 2 metadata columns:
## seqnames ranges strand | key.a key.b
## <Rle> <IRanges> <Rle> | <character> <character>
## [1] chr1 1-7 + | a A
## [2] chr1 1-7 + | a B
## -------
## seqinfo: 1 sequence from an unspecified genome; no seqlengthsThe join_overlap_left() method will perform an outer
left join.
First any overlaps that are found will be returned similar to
join_overlap_inner(). Then any non-overlapping ranges will
be returned, with missing values on the metadata columns.
left_rng <- join_overlap_left(query, subject)
left_rng## GRanges object with 3 ranges and 2 metadata columns:
## seqnames ranges strand | key.a key.b
## <Rle> <IRanges> <Rle> | <character> <character>
## [1] chr1 1-7 + | a A
## [2] chr1 1-7 + | a B
## [3] chr1 9-10 - | b <NA>
## -------
## seqinfo: 1 sequence from an unspecified genome; no seqlengths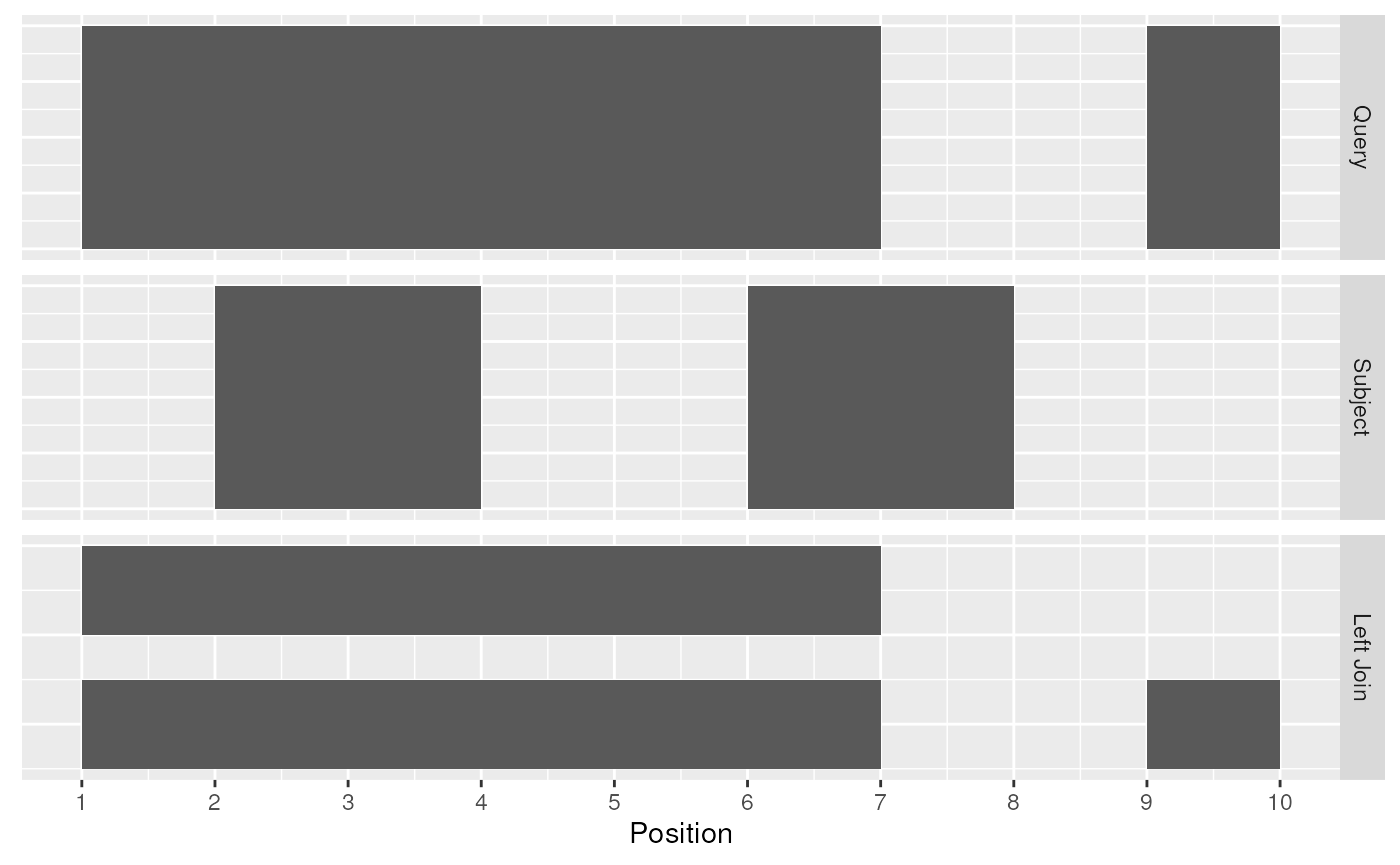
Left Join
Compared with filter_by_overlaps() above, the overlap
left join expands the Ranges to give information about each
interval on the query Ranges that overlap those on the
subject Ranges as well as the intervals on the left that do
not overlap any range on the right.
Finding your neighbours
We also provide methods for finding nearest, preceding or following
Ranges. Conceptually this is identical to our approach for
finding overlaps, except the semantics of the join are different.
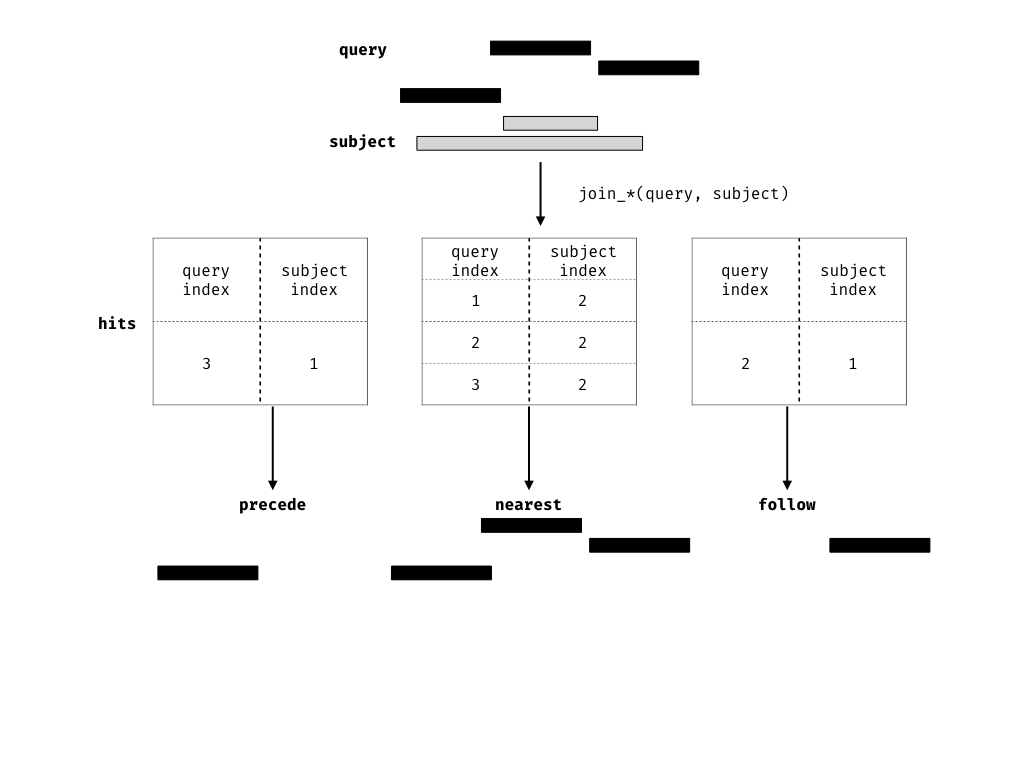
join_nearest(ir0, ir1)## IRanges object with 4 ranges and 1 metadata column:
## start end width | gc
## <integer> <integer> <integer> | <numeric>
## [1] 5 9 5 | 0.780359
## [2] 10 14 5 | 0.780359
## [3] 15 19 5 | 0.780359
## [4] 20 24 5 | 0.780359
join_follow(ir0, ir1)## IRanges object with 4 ranges and 1 metadata column:
## start end width | gc
## <integer> <integer> <integer> | <numeric>
## [1] 5 9 5 | 0.777584
## [2] 10 14 5 | 0.603324
## [3] 15 19 5 | 0.780359
## [4] 20 24 5 | 0.780359
join_precede(ir0, ir1) # nothing precedes returns empty `Ranges`## IRanges object with 0 ranges and 1 metadata column:
## start end width | gc
## <integer> <integer> <integer> | <numeric>
join_precede(ir1, ir0)## IRanges object with 5 ranges and 1 metadata column:
## start end width | gc
## <integer> <integer> <integer> | <numeric>
## [1] 2 4 3 | 0.777584
## [2] 3 6 4 | 0.827303
## [3] 4 8 5 | 0.603324
## [4] 5 10 6 | 0.491232
## [5] 6 12 7 | 0.780359Example: dealing with multi-mapping
This example is taken from the Bioconductor support site.
We have two Ranges objects. The first contains single
nucleotide positions corresponding to an intensity measurement such as a
ChiP-seq experiment, while the other contains coordinates for two genes
of interest.
We want to identify which positions in the intensities
Ranges overlap the genes, where each row corresponds to a
position that overlaps a single gene.
First we create the two Ranges objects
intensities <- data.frame(seqnames = "VI",
start = c(3320:3321,3330:3331,3341:3342),
width = 1) %>%
as_granges()
intensities## GRanges object with 6 ranges and 0 metadata columns:
## seqnames ranges strand
## <Rle> <IRanges> <Rle>
## [1] VI 3320 *
## [2] VI 3321 *
## [3] VI 3330 *
## [4] VI 3331 *
## [5] VI 3341 *
## [6] VI 3342 *
## -------
## seqinfo: 1 sequence from an unspecified genome; no seqlengths
genes <- data.frame(seqnames = "VI",
start = c(3322, 3030),
end = c(3846, 3338),
gene_id=c("YFL064C", "YFL065C")) %>%
as_granges()
genes## GRanges object with 2 ranges and 1 metadata column:
## seqnames ranges strand | gene_id
## <Rle> <IRanges> <Rle> | <character>
## [1] VI 3322-3846 * | YFL064C
## [2] VI 3030-3338 * | YFL065C
## -------
## seqinfo: 1 sequence from an unspecified genome; no seqlengthsNow to find where the positions overlap each gene, we can perform an overlap join. This will automatically carry over the gene_id information as well as their coordinates (we can drop those by only selecting the gene_id).
olap <- join_overlap_inner(intensities, genes) %>%
select(gene_id)
olap## GRanges object with 8 ranges and 1 metadata column:
## seqnames ranges strand | gene_id
## <Rle> <IRanges> <Rle> | <character>
## [1] VI 3320 * | YFL065C
## [2] VI 3321 * | YFL065C
## [3] VI 3330 * | YFL065C
## [4] VI 3330 * | YFL064C
## [5] VI 3331 * | YFL065C
## [6] VI 3331 * | YFL064C
## [7] VI 3341 * | YFL064C
## [8] VI 3342 * | YFL064C
## -------
## seqinfo: 1 sequence from an unspecified genome; no seqlengthsSeveral positions match to both genes. We can count them using
summarise and grouping by the start
position:
## DataFrame with 6 rows and 2 columns
## start n
## <integer> <integer>
## 1 3320 1
## 2 3321 1
## 3 3330 2
## 4 3331 2
## 5 3341 1
## 6 3342 1Grouping by overlaps
It’s also possible to group by overlaps. Using this approach we can count the number of overlaps that are greater than 0.
grp_by_olap <- ir0 %>%
group_by_overlaps(ir1)
grp_by_olap## IRanges object with 6 ranges and 2 metadata columns:
## Groups: query [2]
## start end width | gc query
## <integer> <integer> <integer> | <numeric> <integer>
## [1] 5 9 5 | 0.827303 1
## [2] 5 9 5 | 0.603324 1
## [3] 5 9 5 | 0.491232 1
## [4] 5 9 5 | 0.780359 1
## [5] 10 14 5 | 0.491232 2
## [6] 10 14 5 | 0.780359 2## IRanges object with 6 ranges and 3 metadata columns:
## Groups: query [2]
## start end width | gc query n_overlaps
## <integer> <integer> <integer> | <numeric> <integer> <integer>
## [1] 5 9 5 | 0.827303 1 4
## [2] 5 9 5 | 0.603324 1 4
## [3] 5 9 5 | 0.491232 1 4
## [4] 5 9 5 | 0.780359 1 4
## [5] 10 14 5 | 0.491232 2 2
## [6] 10 14 5 | 0.780359 2 2Of course we can also add overlap counts via the
count_overlaps() function.
ir0 %>%
mutate(n_overlaps = count_overlaps(., ir1))## IRanges object with 4 ranges and 1 metadata column:
## start end width | n_overlaps
## <integer> <integer> <integer> | <integer>
## [1] 5 9 5 | 4
## [2] 10 14 5 | 2
## [3] 15 19 5 | 0
## [4] 20 24 5 | 0Data Import/Output
We provide convenience functions via rtracklayer and
GenomicAlignments for reading/writing the following data
formats to/from Ranges objects.
plyranges functions |
File Format |
|---|---|
read_bam() |
BAM |
read_bed()/write_bed()
|
BED |
read_bed_graph()/ write_bed_graph()
|
BEDGraph |
read_narrowpeaks()/write_narrowpeaks()
|
narrowPeaks |
read_gff() / write_gff()
|
GFF(1-3)/ GTF |
read_bigwig() / write_bigwig()
|
BigWig |
read_wig() /write_wig()
|
Wig |
Learning more
There are many other resources and workshops available to learn to
use plyranges and related Bioconductor packages, especially
for more realistic analyses than the ones covered here:
- The fluentGenomics workflow package is an end-to-end workflow package for integrating differential expression results with differential accessibility results.
- The Bioc
2018 Workshop book has worked examples of using
plyrangesto analyse publicly available genomics data. - The extended vignette in the plyrangesWorkshops package has a detailed walk through of using plyranges for coverage analysis.
- The case study by Michael Love using plyranges with tximeta to follow up on interesting hits from a combined RNA-seq and ATAC-seq analysis.
- The journal article (preprint here) has details about the overall philosophy and design of plyranges.
Appendix
## R version 4.5.2 (2025-10-31)
## Platform: x86_64-pc-linux-gnu
## Running under: Ubuntu 24.04.3 LTS
##
## Matrix products: default
## BLAS: /usr/lib/x86_64-linux-gnu/openblas-pthread/libblas.so.3
## LAPACK: /usr/lib/x86_64-linux-gnu/openblas-pthread/libopenblasp-r0.3.26.so; LAPACK version 3.12.0
##
## locale:
## [1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
## [3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
## [5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
## [7] LC_PAPER=en_US.UTF-8 LC_NAME=C
## [9] LC_ADDRESS=C LC_TELEPHONE=C
## [11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
##
## time zone: UTC
## tzcode source: system (glibc)
##
## attached base packages:
## [1] stats4 stats graphics grDevices utils datasets methods
## [8] base
##
## other attached packages:
## [1] ggplot2_4.0.1 plyranges_1.31.1 dplyr_1.1.4
## [4] GenomicRanges_1.62.0 Seqinfo_1.0.0 IRanges_2.44.0
## [7] S4Vectors_0.48.0 BiocGenerics_0.56.0 generics_0.1.4
## [10] BiocStyle_2.38.0
##
## loaded via a namespace (and not attached):
## [1] SummarizedExperiment_1.40.0 gtable_0.3.6
## [3] rjson_0.2.23 xfun_0.54
## [5] bslib_0.9.0 htmlwidgets_1.6.4
## [7] Biobase_2.70.0 lattice_0.22-7
## [9] vctrs_0.6.5 tools_4.5.2
## [11] bitops_1.0-9 curl_7.0.0
## [13] parallel_4.5.2 tibble_3.3.0
## [15] pkgconfig_2.0.3 Matrix_1.7-4
## [17] RColorBrewer_1.1-3 S7_0.2.1
## [19] desc_1.4.3 cigarillo_1.0.0
## [21] lifecycle_1.0.4 farver_2.1.2
## [23] compiler_4.5.2 Rsamtools_2.26.0
## [25] textshaping_1.0.4 Biostrings_2.78.0
## [27] codetools_0.2-20 htmltools_0.5.8.1
## [29] sass_0.4.10 RCurl_1.98-1.17
## [31] yaml_2.3.10 pillar_1.11.1
## [33] pkgdown_2.2.0 crayon_1.5.3
## [35] jquerylib_0.1.4 BiocParallel_1.44.0
## [37] DelayedArray_0.36.0 cachem_1.1.0
## [39] abind_1.4-8 tidyselect_1.2.1
## [41] digest_0.6.38 restfulr_0.0.16
## [43] bookdown_0.45 labeling_0.4.3
## [45] fastmap_1.2.0 grid_4.5.2
## [47] cli_3.6.5 SparseArray_1.10.1
## [49] magrittr_2.0.4 S4Arrays_1.10.0
## [51] XML_3.99-0.20 withr_3.0.2
## [53] scales_1.4.0 rmarkdown_2.30
## [55] XVector_0.50.0 httr_1.4.7
## [57] matrixStats_1.5.0 ragg_1.5.0
## [59] evaluate_1.0.5 knitr_1.50
## [61] BiocIO_1.20.0 rtracklayer_1.70.0
## [63] rlang_1.1.6 glue_1.8.0
## [65] BiocManager_1.30.27 jsonlite_2.0.0
## [67] R6_2.6.1 MatrixGenerics_1.22.0
## [69] GenomicAlignments_1.46.0 systemfonts_1.3.1
## [71] fs_1.6.6